The “Patients and patents” campaign is led by international patient organizations and aimed at increasing patient community and public understanding of how drug patents are supposed to serve both patients and healthcare systems. The campaign co-leaders are Arthritis Consumer Experts (Canada), the Australian Patient Advocacy Alliance (Australia), and Crohn’s & Colitis Foundation (USA).
About the "Patients and patents" campaign
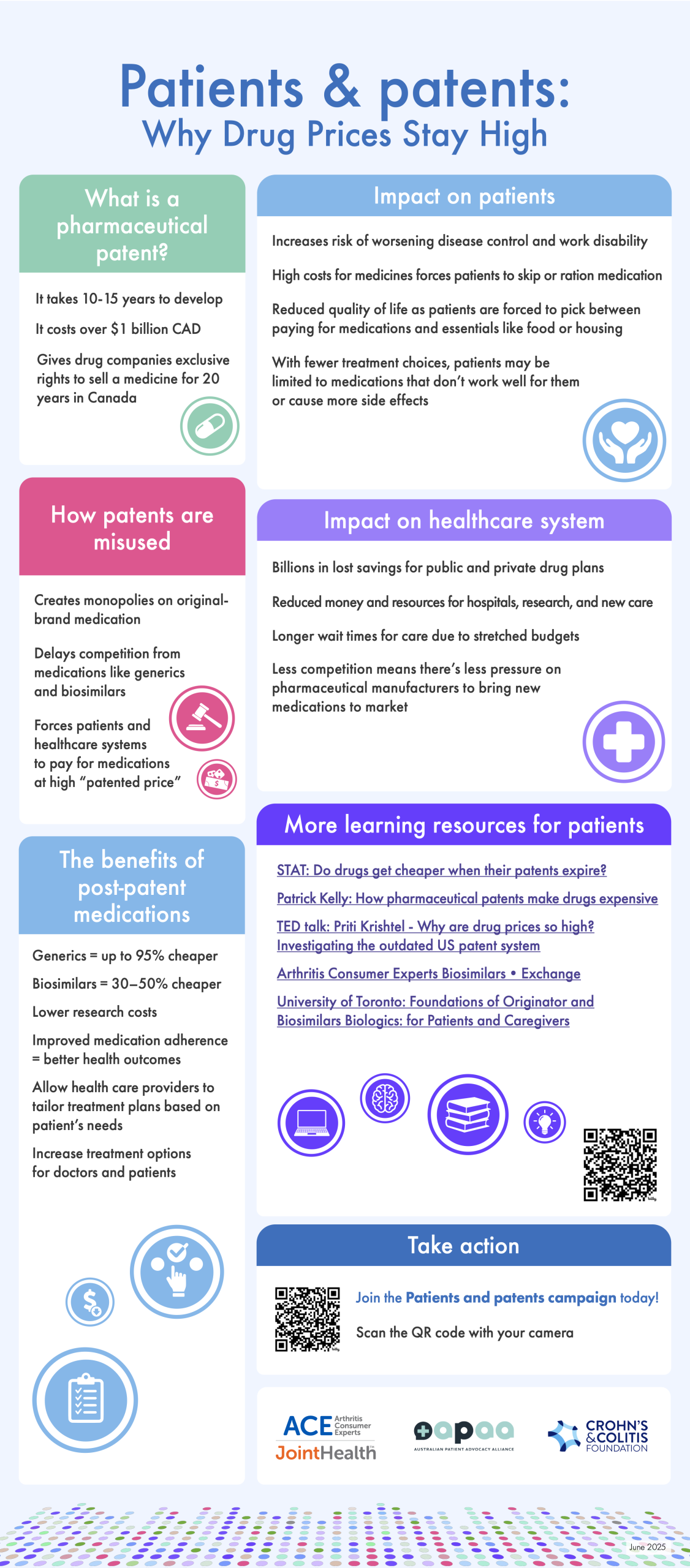
Millions of patients around the world — including those living with arthritis, cancer, inflammatory bowel disease, multiple sclerosis and many other life threatening and chronic diseases — are impacted by the growing costs of prescription medications. One of the drivers of high costs for medications is the misuse of intellectual property (IP) protections by pharmaceutical manufacturers to extend their patent and delay the availability of affordable generic and biosimilar medications.
Understanding how drug patents work in Canada [1]
The “Patients and patents” campaign is led by international patient organizations and aimed at increasing patient community and public understanding of how drug patents are supposed to serve both patients and healthcare systems. The campaign co-leaders are Arthritis Consumer Experts (Canada), the Australian Patient Advocacy Alliance (Australia), and Crohn’s & Colitis Foundation (USA).
A pharmaceutical manufacturer of a new prescription medication spends many years researching and developing a new medication before it can be approved by Health Canada and made available to the public. During this process, one or more patents may be granted to the pharmaceutical manufacturer that developed this new medication.
Patents give pharmaceutical manufacturers the exclusive right to sell a new, original-brand medication in Canada for 20 years from the date of filing.[2] During this time, no other manufacturer is allowed to make, use or sell a generic or biosimilar version of the original-brand medication. This helps the original-brand manufacturer recover the money it spent on research and development.
However, this timeline can be misleading because the patent often gets filed early in the research and development process, long before the medication is approved and ready for patients. By the time the new medication reaches the market, several years of the patent have already passed. This means the company’s real time without competition (called market exclusivity) is often shorter — around 8 to 12 years in Canada.
Generic and biosimilar medications can be made available to the public after Health Canada has approved them as safe and effective. Once approved, generic and biosimilar medications can enter the market at a lower cost (up to 95% for generics and 30–50% for biosimilars), potentially saving healthcare systems billions of dollars. Generic and biosimilar manufacturers do not incur the same early research and development costs to bring the medication to market and can therefore offer it at a lower price.[3]
Key steps in the drug patent process
1. Patent Application:
Pharmaceutical manufacturers file patent applications with the Patent Office, which is part of the Canadian Intellectual Property Office (CIPO).[4]
2. Patent Examination:
The Patent Office reviews the application to determine if the invention is patentable.
3. Patent Grant:
If the application is approved, a patent is granted, giving the company exclusive rights for 20 years from the date of filing.
4. Listing on the Patent Register:
The originator pharmaceutical manufacturer can list the relevant patents on a public Patent Register maintained by the Health Minister.
5. Generic Drug Application and Litigation:
Generic or biosimilar manufacturers can apply to Health Canada for market approval. However, they must address the patents listed on the Patent Register, which can lead to litigation with the original-brand manufacturer.
Responsible parties in the drug patent process
1. Canadian Intellectual Property Office (CIPO):
Responsible for examining patent applications and granting patents.
2. Health Canada:
Regulates the approval of medications, maintains the patent register, and ensures that generic and biosimilar manufacturers address the original-brand manufacturer's patents before receiving market approval.
3. Pharmaceutical Manufacturers (Original-brand and Generic/Biosimilar):
Original-brand manufacturers develop new medications , while generic and biosimilar manufacturers aim to bring more affordable versions to market after patents expire.
The goal of patents
Patents allow the original-brand manufacturer to recover its research and development costs before competitors enter the market with affordable alternatives, like generics and biosimilars.
Developing a new drug is expensive and time-consuming — on average, it takes 10–15 years and costs over $1 billion CAD.
Benefits of patents
- Encourages pharmaceutical manufacturers to invest in the development of new, life- and disability-saving medications.
- Allows pharmaceutical manufacturers to recoup the billions spent on research and development, clinical trials, and regulatory approvals.
- Brings new medications to patients who need them most.
Misuse of patent protection
Pharmaceutical manufacturers can employ legal strategies to extend their patent protection from market competition by applying for additional patents. When filing for these additional patents, pharmaceutical manufacturers might claim they’ve “improved” their formula by changing the non-therapeutic ingredient(s) in their formulation.
By filing for additional patents, manufacturers can create “monopolies” on off-patent original-brand medications, delaying competition from generics and biosimilars medications for years - even decades. These strategies to extend patent protection can result in patients and healthcare systems continuing to pay for medications at the “patented price” rather than at much more affordable off-patent prices.
Key consequences of patent extension for patients
- Higher drug costs: Patients pay higher prices for longer periods, delaying access to more affordable alternatives.
- Financial hardship: Many must choose between paying for medications and other essentials like food or housing.
- Delayed or interrupted treatment: Leads to disease progression, disability, and lower quality of life.
- Reduced adherence: Patients may skip or ration medication due to cost, impacting treatment effectiveness and increasing risk of hospitalizations, surgery and disease progression.
- Emotional distress and mistrust: Patients often feel helpless or betrayed when they learn how the system prioritizes profit over patient care.
Why this matters now
Some pharmaceutical manufacturers are extending patent protection through tactics like “evergreening” (adding patents for minor changes) and “patent thicketing” (filing dozens of overlapping patents) to delay competition far beyond the original protected period.
The "Patients and patents" education campaign
The campaign aims to educate patients, health care providers, policymakers, and the public about how pharmaceutical patents can benefit and potentially harm patients and healthcare systems — especially for chronic disease management that requires timely, continuous access to disability-sparing or life-saving medications.
Additional resources
To learn more, here are additional resources:
- STAT, Do drugs get cheaper when their patents expire, Nov. 4, 2024, available at https://www.youtube.com/watch?v=t-zZFOUa7Vw&t=266s
- Patrick Kelly, How Pharmaceutical Patents Make Drugs Expensive, Aug. 3, 2024, available at https://www.youtube.com/watch?v=nwBKnMONoFM&t=2s
- Priti Krishtel, TED talk. Dec. 2019, available at https://www.youtube.com/watch?v=-3y6_7_5PcQ
How you can get involved
Patients or patient organizations can get involved by sharing our resources. Download, print and share our Campaign Toolkit, including About the Campaign, Question and Answers, Infographic, and Glossary of Key Terms.
Download and share our social media posts, on Facebook, Instagram, X, and LinkedIn - remember to use the #Patientsandpatents hashtag.
[1] Government of Canada: Patents: Learn the basics, October 2024
[2]Paul Grootendorst, P., Bouchard, R., Hollis, A., Canada’s laws on pharmaceutical intellectual property: the case for fundamental reform, Canadian Medical Association Journal, March 2012 184 (5) 543-549: DOI: https://doi.org/10.1503/cmaj.110493
[3] Canadian Agency for Drugs and Technologies in Health (CADTH): Biosimilar Drugs: Your Questions Answered
[4] Government of Canada: Behind the scenes at Canada’s Patent Office, February 2025
Common questions about patents and their impact on patients
About patents
Q1: What are pharmaceutical patents?
A: A pharmaceutical manufacturer may spend years researching and developing a new medication before it can be possibly approved in Canada. Pharmaceutical manufacturers that have developed a new medication often seek a form of legal protection known as a patent right, which gives them the exclusive right to make and sell the medication for a set period without competition. This allows pharmaceutical manufacturers to recover the money they spent on research and development to bring the medication to market.
When the patent expires, generic and biosimilar medications can be made available to the public after Health Canada has approved them as safe and effective. Once approved, generic and biosimilar medications can enter the market at a lower cost (up to 95% for generics and 30–50% for biosimilars), potentially saving healthcare systems billions of dollars.
Q2: Why are generics and biosimilars more affordable than original-brand medications?
A: Developing an originator-brand medication is costly and time-consuming — on average, it takes 10–15 years and costs over $1 billion CAD.
Generic and biosimilar manufacturers do not incur the same early research and development costs to bring the product to market and can therefore offer it at a lower price. [1]
Q3: Why are some medications still so expensive, even though they have been available for decades?
A: Canadians pay some of the highest drug prices in the world. One of the drivers of higher medication costs is the misuse of patent protections by pharmaceutical manufacturers which may delay the availability of affordable generic and biosimilar medications for years, or even decades.
Pharmaceutical manufacturers of original-brand medicines may use legal maneuvers such as “evergreening” (adding patents for minor changes) and “patent thicketing” (filing dozens of overlapping secondary patents) to extend their market monopoly.
Example: Humira (adalimumab) – used to treat inflammatory forms of arthritis and inflammatory bowel disease.
- The original patent expired in 2017, but the manufacturer filed dozens of additional patent applications, extending market monopoly until 2021 in Canada.
- Total cost of this delay: Over $1 billion in additional drug spending for Canada’s public drug plans.
Q4: How do original-brand manufacturers delay competition for off-patent generic and biosimilar medications entry into the market?
A: Manufacturers use patent strategies (e.g. secondary patents on formulations, dosages or delivery methods) to extend their market monopoly, which block competition and keep their medication at the “patented price.”
Common tactics used to delay affordable medications:
- Evergreening: Filing new patents on minor changes (e.g., different dosages, coatings, or packaging) to extend exclusivity.
- Patent Thickets: Flooding the system with dozens of overlapping patents to create legal and financial barriers for competitors.
- Patent Linkage System: In Canada, the regulatory approval of generic/biosimilar medicine can be delayed automatically by 24-month if the original-brand manufacturer list one or more patents on the Patent Register and asserts these patents against the generic or biosimilar manufacturers, even if the patents are likely invalid or not infringed.
Q5: How does delayed access to generics and biosimilars impact patients?
A: Most people living with inflammatory arthritis like rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and lupus require lifelong medications to prevent permanent joint damage.
While safe and effective, Health Canada approved generics and biosimilars are blocked from entering the market due to the drug patents, and patients have limited treatment options. Delayed access to affordable treatment options can have negative effects on health outcomes:
- Without affordable alternatives, patients must use higher cost original-brand medications or go without treatment.
- Delayed or interrupted treatment gaps can lead to disease progression, disability and lower quality of life.
High medication prices can also lead to inconsistent treatment adherence, resulting in poor health outcomes.
- Example: A person with inflammatory arthritis who cannot afford their advanced therapy may skip treatment or ration or take lower doses to make their prescriptions last longer or stop taking the medication altogether.
- Reduced medication adherence can cause worsening symptoms, increased pain, loss of mobility, hospitalization, surgery, loss of job, or permanent joint damage.
Q6: How does misuse of patent protection affect out-of-pocket medication costs for patients?
A: One in four Canadians currently cannot afford their prescription medications. Misuse of patent protection can result in patients continuing to pay for medications at the “patented price” rather than at much more affordable post-patent prices. Higher out-of-pocket medication costs have multiple impacts for patients:
- Patients pay for high-priced medications for years longer than necessary.
- Some patients must choose between paying for medicine or necessities like food, rent, or utilities.
- Financial hardship leads to debt, stress, and sacrificing other aspects of care.
- Patients often feel helpless or betrayed when they learn how the system prioritizes profit over patient care.
Q7: What are the emotional and psychological effects of high medication prices?
A: Patients who cannot afford their medication may feel forced to choose between their health and their financial stability. The constant financial strain creates stress, anxiety, and feelings of helplessness. Many patients experience guilt or distress if they must ask for financial help to afford medication.
Q8: How does misuse of patent protection increase healthcare costs for everyone?
A: Misuse of patent protection keeps healthcare costs high by forcing public and private drug plans to pay for medications at the “patented price” rather than at lower post-patent prices. Private health insurers may pass these higher medication costs onto patients through increased premiums and co-pays. Additionally, public drug plans may have less funding for new medications and other essential health services for people living with a chronic disease like inflammatory arthritis.
Q9: Does the misuse of patent protection affect the development of new drugs?
A: Yes, manufacturers may prioritize extending old drug patents over researching and developing new medications. In this case, patients may lose access to potential treatment breakthroughs.
If pharmaceutical manufacturers focused on innovation rather than patents, patients could potentially see more new and effective treatments enter the market, and more quickly.
About the "Patients and patents" campaign
Q10: Is there a need for a patient organization-led education campaign on pharmaceutical patents?
A: The percentage of patients who fully understand pharmaceutical patent law is generally quite low. Various studies and surveys suggest that the public, including patients, have limited knowledge and understanding of pharmaceutical patents and their impact on patients.
The campaign aims to deliver evidence-based education on pharmaceutical patents and demonstrate that while they help drive research and innovation, they can also affect patient health and access to affordable medications.
Q11: Who is leading the "Patients and patents" campaign?
A: The "Patients and patent" campaign is being co-led by:
- Arthritis Consumer Experts (Canada)
- Australian Patient Advocacy Alliance (Australia)
- Crohn’s & Colitis Foundation (USA)
Please feel free to download, print, and share this material.
[1] Canadian Agency for Drugs and Technologies in Health (CADTH): Biosimilar Drugs: Your Questions Answered
The "Patients and patents" campaign infographic
The "Patients and patents" campaign infographic details what a pharmaceutical patent is and looks at some misuses of patent protection and the impact these misuses may have on patients and the healthcare system. The infographic is available digitally, is downloadable, and can be printed front and back on 8 ½” x 11” paper.
Click here to download the infographic.
Join the "Patients and patents" conversation online
Patient organizations and individual patients can get involved with the "Patients and patents" campaign by sharing its resources and infographic. We have created a social media kit with sample social media posts and graphics for Facebook, Instagram, X, BlueSky and LinkedIn. For all posts, please remember to use the hashtag #Patientsandpatents and tag the following organizations on their respective channels:
- Arthritis Consumer Experts (Canada): Website | Facebook | Instagram | X | BlueSky | LinkedIn | YouTube
- Australian Patient Advocacy Alliance (Australia): Website | LinkedIn
- Crohn’s & Colitis Foundation (USA): Website | Facebook | Instagram | X | BlueSky | TikTok | LinkedIn | YouTube
Click here to download the social media kit.
Glossary of patent terminology
Pharmaceutical Patent
A form of legal protection that gives a pharmaceutical manufacturer the exclusive right to sell a new original-brand medication for a certain period; in Canada, 20 years from the date of filing.[1] During this time, no other manufacturer is allowed to make, use or sell a generic or biosimilar version of the original-brand medication. This helps the original-brand manufacturer recover the money it spent on research and development and to profit from their discovery for the life of the patent.
Primary & Secondary Patents
A primary patent protects the original active ingredient of a medication. This is the strongest form of patent protection.
Secondary patents often cover small modifications to the medication, such as:
- New formulations (e.g., from a tablet to an extended-release version)
- New delivery methods (e.g., an injection instead of a pill)
- New combinations (mixing two old drugs together)
Original-brand pharmaceutical manufacturers file multiple secondary patents to block generic or biosimilar entry. Many secondary patents offer little or no new medical benefit, but they extend the “patented price” of the medication.
Original-brand Medication
The first version of a medication Health Canada has approved for sale is known as the original-brand medication.
Generic Medications
Generic medications such as aspirin or ibuprofen are small molecules that are chemically synthesized. They contain identical active ingredients as the original-brand medications and are considered the same in terms of dosage form, safety, effectiveness and intended use. Generic medications incur significantly less developmental costs and are offered at a significantly less price than an original-brand drug medication. For example, in Canada, the generic version of ibuprofen costs approximately 90% less than its original-brand medications (Advil, Motrin).
Biosimilar Medications
Biologics are made from living organisms like yeast and bacteria and are much larger and more complex in nature than conventional, small molecule medicines such as over-the-counter ibuprofen or by-prescription methotrexate.
As patents expire for original-brand biologic medicines, other manufacturers produce biologic medicines that are called biosimilars. To receive Health Canada’s approval, a biosimilar must demonstrate it is as safe and effective as the original-brand. Manufacturers that make biosimilars do not incur the same developmental costs in order to bring the biosimilar to market and will therefore offer it at a lower price (up to 30-50% in Canada).
Biosimilars have been approved for use in Canada since 2009 and for use in inflammatory arthritis since 2014. Sixty-four biosimilars are currently approved by Health Canada.
Patent Exclusivity in Canada
A legal right that prevents others from making, selling, or using a medication protected by patent rights until the expiry of the last relevant patent. Unlike regulatory exclusivity, which is automatic, patent exclusivity depends on filing and maintaining patents and the outcome of any subsequent patent litigation.
How it works in Canada:
- The legal right of the primary patent (on the active ingredient) lasts for a certain period of time.
- Although the patent term in Canada is 20 years from the date of filing, there is a critical distinction between this basic patent timeline and the length of a drug’s monopoly: for drug patents, the patent clock starts ticking as soon as the application is filed, which typically occurs early in the drug research and development process. This means a significant portion of the patent term may elapse before the drug ever reaches the market, reducing the manufacturer’s real time without competition (called market exclusivity) - approximately 8-12 years in Canada.
- Secondary patent rights (on formulations, dosages, or delivery methods) can extend the period of market monopoly.
- If a generic or biosimilar manufacturer challenges a patent listed on the Patent Register, the patent linkage system delays the regulatory approval of that generic or biosimilar for an automatic 24-months.
Why it matters:
- Manufacturers file multiple secondary patents to create a "patent thicket", extending their monopoly.
- Even if the original-brand patent expires, secondary patents can block generics for years.
Example: The drug Humira (adalimumab) had a primary patent that expired in 2017, but the manufacturer filed dozens of additional patents, keeping biosimilars off the Canadian market until 2021.
Regulatory Exclusivity
A government-granted period of time during which a pharmaceutical manufacturer has exclusive rights to its clinical trial data. Even if a drug’s patent has expired, generics and biosimilars cannot apply for approval until this exclusivity period ends.
How it works in Canada:
- New innovative medicines get 8 years of regulatory exclusivity before generics or biosimilars can be approved.
Why it matters:
- Generic and biosimilar manufacturers cannot reference the original-brand medication’s clinical data to get approval for their generic or biosimilar medicines during this period.
- This delays competition even if there are no active patents on the medication.
Example: A biologic medicine approved in 2020 will have regulatory exclusivity until 2028, meaning no biosimilar can obtain regulatory approval until that year — even if the drug’s patent expired in 2025.
Evergreening
A tactic used by pharmaceutical manufacturers to extend a drug’s patent beyond its original 20-year limit by making minor changes and filing new patents. This delays generics and biosimilars from entering the market. For example, a company may change the dosage form (from a tablet to an extended-release capsule) or create a new injection device to get a new patent and extend its monopoly on the medication.
Patent Thicket
A strategy where a pharmaceutical manufacturer files dozens (or even hundreds) of overlapping patents on a single medication to make it harder for generics and biosimilars to enter the market.
Patent Linkage System
A legal system in Canada that automatically blocks a generic or biosimilar medication from being approved for 24 months if the original-brand manufacturer asserts a patent listed on the Patent Register.
Please feel free to download, print, and share this material.
[1] Paul Grootendorst, P., Bouchard, R., Hollis, A., Canada’s laws on pharmaceutical intellectual property: the case for fundamental reform, Canadian Medical Association Journal, March 2012 184 (5) 543-549: DOI: https://doi.org/10.1503/cmaj.110493
Stay informed
Get the latest arthritis news and updates from ACE directly to your inbox.

Arthritis Consumer Experts
© 2000-2025


